What
Is pH?
pH
(the power, or potential, of Hydrogen)
= the unit that indicates the hydrogen density of acid and alkaline
water.
Water
becomes acidic with increased hydrogen ions (H+), and alkaline
with increased hydroxyl ions (-OH). The measuring unit of acidity
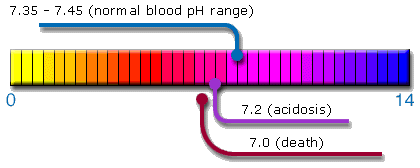
and alkalinity is indicated by pH values, e.g., pH 7 is considered
neutral. Each numerical increment indicates 10 times more hydrogen
or hydroxyl ions. For example, an increase of 1 in a pH table
means 10 times more alkalinity. A decrease of 1 means 10 times
more acidity.
| The
pH level of our internal fluids affects every cell in our
bodies. The entire metabolic process depends on an alkaline
environment. Chronic over acidity corrodes body tissue,
and if left unchecked will interrupt all cellular activities
and functions, from the
beating of your heart to the neutral firing of your brain.
In other words, overacidity interferes with life itself.
It is at the root of all sickness and disease.
|
2)
Maintaining pH balance
The
human body tends to maintain body fluid (blood) at pH 7.365, according
to the homeostasis function. The body tries to maintain the balance
of each organ with alkaline body fluid of pH 8.8 from the pancreases
as the peak. However, consistent acidic constitution will result
in lowering one's self-protection function in the body. Even though
there is no visible sign of disease, alkaline water drinking will
support the body with homeostasis functions. Drinking alkaline
water also prevents geriatric disease because it defends the body
against acidification of the physical constitution.
|